Ozone O3 Is a Form of Elemental Oxygen
Ozone O3 is not generally referred to as elemental oxygen but as an allotrope of oxygen O2. Write the unbalanced equation for the formation of ozone gas from oxygen gas.
Is Ozone Counted As An Element Quora
ἄλλος allos other and τρόπος tropos manner is a behavior exhibited by certain.

. Ozone is produced from atmospheric oxygen gas O2 by the high energy outbursts found in lightening storms. It is a pale blue gas and consists of three oxygen atoms. Correct answer to the question Ozone o3g is a form of elemental oxygen that is important in the absorption of ultraviolet radiation in the stratosphere.
Express your answer to four. Ozone O3 g is a form of elemental oxygen that is important in the absorption of ultraviolet radiation in the stratosphere. Ozone is an allotropic molecular form of oxygen containing three atoms of oxygen O 3.
It is a triatomic three-atom molecule consisting only of the element oxygen. Molecular oxygen is inherently more stable than ozone but there is so much UV light in the stratosphere that it creates an equilibrium level of about 5 parts per million ppm or about 00005 ozone. Ozone O3 g is a form of elemental oxygen produced during electrical discharge.
Ozone is produced from atmospheric oxygen gas O2 by the high energy outbursts found in lightening storms. Ozone O3 9 is a form of elemental oxygen that is important in the absorption of ultraviolet radiation in the stratosphere. Include states-of-matter under SATP conditions in your answer.
Ozone O3g is a form of elemental oxygen that is important in the absorption of ultraviolet radiation in the stratosphere. Ozone O3 is generated through the passage of oxygen O 2 through a high voltage potential resulting in the attachment and formation of a third oxygen atom. And pressure according to the following reaction.
G is a form of elemental oxygen that plays an important role in the absorption of ultraviolet radia tion in the stratosphere. Closer to home lightning makes ozone. Questions in other subjects.
Ozone O 3 g O_3 g O3. Any element that exists naturally in the uncombined state as the free element eg gold may be referred to as elemental. Ozone is used to deodorize air purify water and treat industrial wastes.
O 2 g O_2 g O2. Is δhf for o3g. Up to 256 cash back Ozone O 3 g is a form of elemental oxygen that plays an important role in the absorption of ultraviolet radiation in the stratosphere.
It decomposes to O2g at room temperature and pressure according to the following reaction. 203 9302 9 AH -2846 kJ Part A What is the enthalpy change for this reaction per mole of O3 9. It decomposes to O2g at room temperature and pressure according to the following reaction.
Ozone o3g is a form of elemental oxygen produced during electrical discharge. Write the unbalanced equation for the formation of ozone gas from oxygen gas. Ozone has pungent an odor and its color is blue-black in its solid and liquid form.
It decomposes to O2 g at room temperature and pressure according to the following reaction. Ozone O 3 g is a form of elemental oxygen that plays an important. 2O3g 3O2gΔH -2846 kJ.
2 O 3 g ------- 3 O 2 g ΔH -2846 kJ. Ozone gas is a form of elemental oxygen containing molecules with three oxygen atoms O3. Formed in the ozone layer of the stratosphere it is harmful to life.
A34 of state legislatures ratify a proposed amendment B34 of states holding conventions ratify a proposed amendment C. In the stratosphere ozone O3 can be made by the interaction of UV light with molecular oxygen O2. Ozone O3g is a form of elemental oxygen that is important in the absorption of ultraviolet radiation in the stratosphere.
Include states-of-matter under SATP conditions in your answer. It decomposes to O 2 g at room temperature. Ozone gas is a form of elemental oxygen containing molecules with three oxygen atoms O3.
In order to understand the difference between oxygen and ozone it is important to understand what an allotrope is. Ozone is a powerful oxidizing allotropic form of oxygen. Ozone O 3 is an allotrope of oxygen.
Ozone O 3 is a triatomic molecule consisting of three oxygen atomsIt is an allotrope of oxygen that is much less stable than the diatomic O 2 oxygen gas. It decomposes to O2 g at room temperature and pressure according to the following reaction 203 g-3029 AH-2846 kJ What is the enthalpy change for this reaction per mole of O3 g. It decomposes to O 2 g at room temperature and pressure according to the following reaction.
Role in the absorption of ultraviolet radiation in the. Is ozone an element or a molecule. Which is not a way to formally amend the US Constitution.
However ozone may be referred to as an elemental molecule by virtue of it being composed solely of O atoms.

Solved Ozone Mathrm O 3 G Is A Form Of Elemental Oxygen That Is Important In The Absorption Of Ultraviolet Radiation In The Stratosphere It Decomposes To Mathrm O 2 G At Room Temperature And Pressure According To The
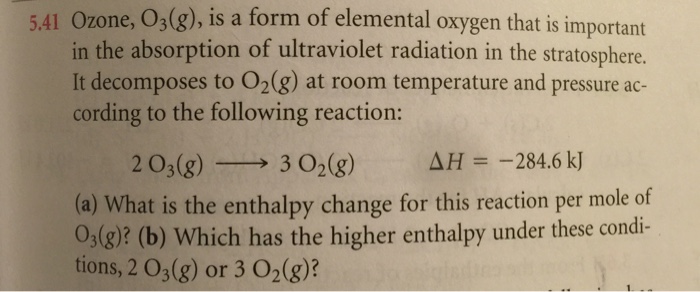
Solved Ozone O 3 G Is A Form Of Elemental Oxygen That Is Chegg Com

Oxygen O2 And Ozone O3 Molecule Models And Chemical Formulas Dioxygen And Trioxygen Gas Ball And Stick Models Geometric Structures And Structural Formulas Illustration On White Background Vector Royalty Free Svg Cliparts Vectors And

Natural Resources Chapter Notes Natural Resources Chapter Resources
Comments
Post a Comment